
We always start with a risk analysis, to investigate risks and determine the most important functions for testing. We are able to guide your company during validation processes, or completely carry out your project from A to Z, including testing and writing documents like user requirements. Most companies may rely on the GAMP checklist to evaluate their systems even though the current business environment requires thorough validation process. We use cookies to optimise your experience on our website. For users, GAMP guideline gives the principles that assure that the automated system is appropriate for the intended use before the pharmaceutical products are produced while the suppliers are guided by GAMP to check and test any avoidable defects in the system hence ensuring the products supplied by the pharmaceutical industry meets high-quality standards.Įach set of guidelines outline legal requirements for the chemical content of each grade of water, including a three stage conductivity test for inorganic compounds that will determine pH and total organic oxygen TOC levels. The use of independent recorders for monitoring the autoclave gives confidence that the process has performed as required and is usually part of the product release documentation. GAMP recognizes two levels of hardware Hardware Category 1 – Standard Hardware Components The majority of the hardware used by regulated companies will fall into this category. Can’t read the image? Although this document has no legal standing and is purely advisory, it does contain information and methodologies that are of interest to anyone engaged in validation activities within the cGMP regulated environment. Good automated manufacturing practice GAMP is both a technical subcommittee of the International Society for Pharmaceutical Engineering ISPE and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry.Ĭomments shall be published after review. This way, we work in the most efficient way possible and we help you focus on product quality and patient safety while reducing validation costs.įor operators of a storage plant, it is necessary not only to ensure that products are stored at the right temperature, but also that the refrigeration plant is capable of accurately maintaining that temperature.
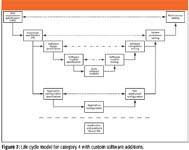
The approach to supplier assessment should be riskbased and documented. Stay up to date on branche news and new QbD services, products and information. Hardware Category 1 – Standard Hardware Components The majority of the hardware used by regulated companies will fall into this category. The strategies Many of the guidelines in GAMP®5 come down to common sense. GAMP 5 should be implemented for the automated systems in pharmaceutical manufacturing and quality control to produce the high quality. The new GAMP-5 guidelines were released February at the ISPE Manufacturing Excellence Conference in Tampa, Florida.


 0 kommentar(er)
0 kommentar(er)
